Model |
ICM09 |
ICM09B |
ICM09JB |
Polarity |
Unipolar |
Bipolar |
Bipolar |
Shape |
Straight |
Straight |
J-Shape |
Chamber |
Ventricle/Atrium |
Ventricle/Atrium |
Atrium |
Fixation |
4 tines |
4 tines |
4 tines |
Material |
|||
Inner insulation |
- |
Silicone MED 4719 |
Silicone MED 4719 |
| Outer insulation | Polyurethane (55D) | Polyurethane (55D) | Polyurethane (55D) |
Conductor |
MP 35 N nickel alloy |
MP 35 N nickel alloy |
MP 35 N nickel alloy |
Tip electrode |
Titanium nitride coated platinum alloy |
Titanium nitride coated platinum alloy |
Titanium nitride coated platinum alloy |
Ring electrode |
- |
Titanium nitride coated platinum alloy |
Titanium nitride coated platinum alloy |
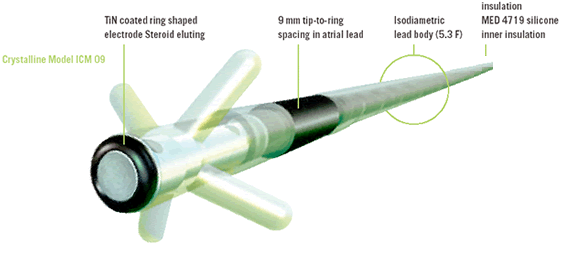
| Body diameter | 1.2 mm ( 3.5 F ) | 1.8 mm (5.3 F ) | 1.8 mm ( 5.3 F ) |
Introducer size |
|||
One lead |
7 F |
7 F |
7 F |
With guide wire |
8 F |
9 F |
9 F |
| Tip/Ring distance | |||
| Tip/Ring distance | - | 17 mm | 9mm |
Electrode surface area |
|||
Tip |
2.5 mm² |
2.5 mm² |
2.5 mm² |
Ring |
- |
24 mm² |
24 mm² |
Coil resistance |
|||
Unipolar |
40Ω (58 cm) |
41Ω (58 cm) |
37Ω (53 cm) |
Bipolar |
- |
83Ω (58 cm) |
51Ω (53 cm) |
Steroid |
< 1.0 mg dexamethasone acetate and dexamethasone sodium phosphate |
< 1.0 mg dexamethasone acetate and dexamethasone sodium phosphate |
< 1.0 mg dexamethasone acetate and dexamethasone sodium phosphate |
Standard lengths |
52, 58, 65 cm |
52, 58, 65 cm |
45, 53 cm |
Connector |
IS-1 UNI |
IS-1 BI |
IS-1 BI |
Serial number prefix |
VMS |
VMT |
VMU |

Intended Use
Vitatron implantable transvenous leads are designed to be used with pulse generators as part of a cardiac pacing system. See product labeling for specific directions regarding application.
Contraindications
When tricuspid valvular disease is present, use of a transvenous ventricular lead is contraindicated. In patients with mechanical tricuspid heart valves, use of endocardial ventricular leads is contraindicated. Use of a passive fixation atrial lead may be contraindicated in the absence of a right atrial appendage. Do not use steroid eluting leads in patients for whom a single dose of 1.0 mg dexamethasone sodium phosphate may be contraindicated.
Warnings
An implanted lead forms a direct, low-resistance current path to the myocardium. Therefore, use only battery-powered equipment during lead implantation and testing to protect against fibrillation caused by alternating currents. Line-powered equipment used in the vicinity of the patient must be properly grounded. Lead connector pins must be insulated from any leakage currents that may arise from line-powered equipment.
Precautions
Use an anchoring sleeve with all leads. Leads should be handled with great care at all times. Any severe bending, kinking, stretching, handling with surgical instruments or excessive force when inserting a stylet may cause permanent damage to the lead. Chronic repositioning may adversely affect a steroid lead's low-threshold performance.
Potential Complications
The potential complications related to the use of transvenous leads include, but are not limited to the following patient-related conditions that can occur when the lead is being inserted and/or repositioned: valve damage (particularly in fragile hearts, e.g., infants), fibrillation and other arrhythmias, thrombolytic and air embolism, cardiac perforation, heart wall rupture, cardiac tamponade, muscle or nerve stimulation, pericarditis, pericardial rub, infection, myocardial irritability, thrombosis, and pneumothorax.